Calculations of Volumetric analysis
The following points should be reminded while making calculations of volumetric exercises.
(i) 1g equivalent mass of a substance performs completely with 1g equivalent mass of any other substance. 1g equivalent mass of a substance means equivalent mass of the substance in grams. For example,
1g equivalent mass of NaOH = 40 g of NaOH
1g equivalent mass of H2SO4 = 49 g of H2SO4
1g equivalent mass of KMnO4 in acidic medium = 31.6 g of KMnO4
1g equivalent mass of hydrated oxalic acid = 63 g of hydrated oxalic acid
Note : Equivalent mass is a variable quantity and depends on the reaction in which the substance takes part. The nature of the reaction should be known before writing the gram equivalent mass of the substance. For example in the reactions.
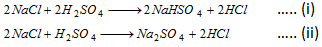
The value of g equivalent mass of H2SO4 in reaction (i) is 98 g and in reaction (ii) 49g.
(ii) Number of g equivalents
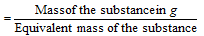
Number of g moles 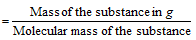
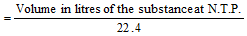 (only for gases)
(only for gases)
Number of milli-equivalent 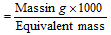
Number of milli-moles 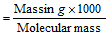
(iii) Molarity 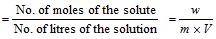
Molarity * molecular mass = strength of the solution (g/L) No. of moles of the solute = Molarity * No. of litres of solution Mass of the solute in g(w) =molarity * No. of litres of solution * mol. mass of solute
Normality 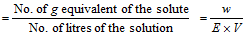
Normality * equivalent mass = strength of the solution (g/L)
No. of equivalents of the solute = Normality * No. of litres of solution
Mass of the solute in g(w) = Normality * No. of litres of solution * Eq. mass of the solute
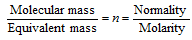
Normality = n * Molarity
(iv) Normality equation : When solutions A and B react completely.
NAVA = NBVB
Normality of A * volume of A = Normality of B * volume of B
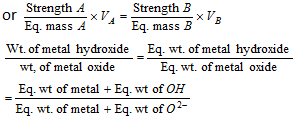
(v) When the solution is diluted, the following formulae can be applied:
N1V1 = N2V2 or M1V1 = M2V2 or S1V1 = S2V2
Before dilution = After dilution
(vi) If a number of acids are mixed, the combined normality of the mixture, Nx, is provided
NxVx = N1V1 + N2V2 + N3V3 +.......
Where Vx is the total volume of the mixture, N1 and V1 are the normality and volume respectively of one acid, N2 and V2 of the second acid and so on.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Calculations of Volumetric analysis questions? Calculations of Volumetric analysis topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Calculations of Volumetric analysis related problems. We provide step by step Calculations of Volumetric analysis question's answers with 100% plagiarism free content. We prepare quality content and notes for Calculations of Volumetric analysis topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours