Molecular formula : Molecular formula of a substance gives the actual number of atoms present in one molecule of the substance.
Molecular formula = n * Empirical formula
Where, n is a simple integer 1, 2, 3,...... etc. defined by the equation,
n = molecular mass of compound / Empirical Formula Mass of the compound
where the molecular mass of the compound is determined experimentally by any one of the methods discussed former, empirical formula mass is calculated by adding the atomic masses of all the atoms present in the empirical formula.
Molecular formula of gaseous hydrocarbons (Eudiometry)
Eudiometry is a direct process for calculation of molecular formula of gaseous hydrocarbons without determining the percentage composition of various elements in it and without knowing the molecular weight of the hydrocarbon. The actual method used involves the following steps,
(a) A known volume of the gaseous hydrocarbon is mixed with an excess (known or unknown volume) of oxygen in the eudiometer tube kept in a trough of mercury.
(b) The mixture is exploded by passing an electric spark between the platinum electrodes. As a result, hydrogen and carbon of the hydrocarbon are oxidised to CO2 and H2O vapours respectively.
(c) The tube is given to cool at room temperature when water vapours condense to give liquid water which has a negligible volume as compared to the volume of water vapours, Thus, the gaseous mixture left behind in the eudiometer tube after explosion and cooling consists of only CO2 and unused O2.
(d) Caustic potash or caustic soda solution is then introduced into the eudiometer tube which absorbs CO2 completely and only unused O2 is left behind. 
Thus, the decrease in volume on introducing NaOH or KOH solution gives the volume of CO2 formed. Sometimes, the volume of O2 left unused is found by introducing pyrogallol and noting the decrease in volume.
Calculation : From the volume of CO2 formed and the total volume of O2 used, it is possible to calculate the molecular formula of gaseous hydrocarbon with the help of the following equation.
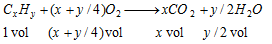
(Negligible volume on condensation)
From the above equation, it is evident that for one volume of hydrocarbon,
(a) (x+y/4) volume of O2 is used
(b) x volume of CO2 is produced
(c) y/2 volume of H2O vapours is produced which condense to give liquid H2O with negligible volume.
(d) Contraction on explosion and cooling
= [(1 + x + y/4) - x] = 1 + y/4
By calculating the experimental results with the theoretical values from the above combustion equation, the values of x and y and hence the molecular formula of the gaseous hydrocarbon can be easily determined.
Calculation of structure by spectroscopic and diffraction methods : The structures of organic substances are determined by spectroscopic and diffraction methods.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Calculation of Molecular Formula questions? Calculation of Molecular Formula topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Calculation of Molecular Formula related problems. We provide step by step Calculation of Molecular Formula question's answers with 100% plagiarism free content. We prepare quality content and notes for Calculation of Molecular Formula topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours