Boron
Boron is the first member of group -13 (IIIA) of the periodic table. Boron is a non- metal. It has a small size and high ionization energy due to which it cannot lose its valence electrons to form B+3 ion. Its compounds especially the hydrides and halides are electron deficient and behave as Lewis acid.
(1) Ores of boron
(i) Borax or tincal : Na2 B4O7 . 10H2O
(ii) Kernite or Rasorite : Na2 B4O7 . 4H2O
(iii) Colemanite : Ca2 B6O11 . 5H2O
(iv) Orthoboric acid : H3BO3 (It occurs in the jets of steam called soffioni escaping from ground in the volcanic region of the Tuscany). The boron is present in a very small extent (0.001%) in earth's crust.
(2) Isolation : Elemental boron in the form of dark brown powder is obtained either by reduction of boric oxide with highly electropositive metals such as Mg, K, Na, Al, and more in the absence of air and boron halides with hydrogen at high temperature for e3xample
B2O3 + 6K  2B + 3K2O
2B + 3K2O
2BCl3 + 3H2 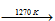 2B + 6HCl.
2B + 6HCl.
By thermal decomposition of boron triiodide over red hot tungsten filament and boron hydrides for example,
2BI3 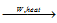 2B + 3I2 ; B2H6
2B + 3I2 ; B2H6  2B + 3H2
2B + 3H2
(3) Properties : It exists in mainly two allotropic forms i.e. amorphous dark brown powder and crystalline black very hard solid. It occurs in two isotopic forms, i.e., 5B10 (20% abundance) and 5B11 (80% abundance). With air, boron forms B2O3 and BN at 973K, with halogens, trihalides (BX3) are formed, with metals borides are formed for example,
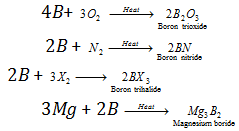
Water, steam and HCl have no action on B. oxidising acids (HNO3, H2SO4) convert boron to H3BO3.
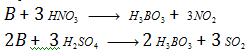
Fused alkalies (NaOH, KOH) dissolve boron forming borates, liberating hydrogen.
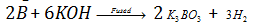
(4) Uses of Boron : Boron is used in atomic reactors as protective shields and control rods, as a semiconductors for making electronic devices in steel industry for increasing the hardness of steel and in making light composite materials for air craft's.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Boron and its compounds questions? Boron and its compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Boron and its compounds related problems. We provide step by step Boron and its compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for Boron and its compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours