Boric acid or orthoboric acid (H3BO3)
It is obtained from borax by treating with dilute HCl or dillute H2SO4 ,
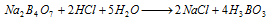
It can be obtained from the mineral colemanite as well by passing SO2 through a mixture of powdered mineral in boiling water,
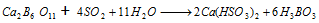
Properties : (a) It is a very weak monobasic acid, do not act as the proton doner but behaves as a Lewis acid that is it accepts a pair of electrons from OH- ion of H2O ,

It acts as a strong acid in presence of polyhydroxy compounds like mannitol glycerol, etc. and can be titrated against strong alkali .
(b) With the NaOH it forms, sodium metaborate,

(c) With and conc. , it gives triethyl borate
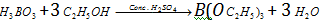
(d) Action of heat : The complete action of heat on boric acid may be written as,
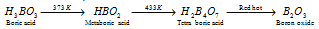
Structure : In boric acid, planar BO3-3 units are joined by hydrogen bonds to give a layer structure.
Uses : (a) As a food preservative. (b) As a mild antiseptic for eye wash under the name boric lotion. (c) For the preparation of glazes and enamels in pottery.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Boric Acid questions? Boric Acid topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Boric Acid related problems. We provide step by step Boric Acid question's answers with 100% plagiarism free content. We prepare quality content and notes for Boric Acid topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours