Borax (Na2B4O7.10H2O)
It occurs naturally as tincal (Suhaga) which contains about 50% borax in certain land, lakes. It is also obtained from the mineral colemanite by boiling it with a solution of Na2CO3.
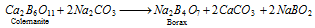
Properties : (a) Its aqueous solution is alkaline due to hydrolysis,
Na2B4O7 + 7H2O → 2NaOH+4H3BO3.
(b) On heating borax loses its water of crystallization and swells up to form a fluffy mass. On further heating, it melts to give a clear liquid which solidifies to a transparent glassy bead consisting of sodium metaborate (NaBO2) and boric anhydride (B2O3),
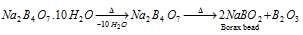
Borax bead is used for the detection of coloured basic radicals under the name borax bead test.
Borax bead test : Borax bead is a mixture of NaBO2 and B2O3.B2O3 on heating combines readily with a number of coloured transition metal oxides such as Ni, Co, Mn, Cr, Cu, etc. to form corresponding metaborates which possess characteristic colours,
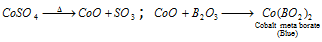
Colours of some significant metaborates are: Cupric metaborate, Cu(BO2)2 is dark blue, chromium metaborate, Cr(BO2)2 is green, nickel metaborate, Ni(BO2)2 is brown and manganese metaborate, Mn(BO2)2 is pink violet.
When heated with C2H5OH and conc.H2SO4, it gives volatile vapours of triethyl borate which burns with a green edged flame.
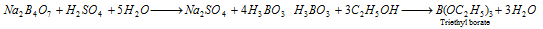
This type of reaction is used as a test for borate radical in the qualitative analysis.
Uses :
(a) In the making optical and hard glasses.
(b) In laboratory for the borax bead test.
(c) In softening of the water.
(d) In preparation of the medicinal soaps because of its antiseptic character.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Borax questions? Borax topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Borax related problems. We provide step by step Borax question's answers with 100% plagiarism free content. We prepare quality content and notes for Borax topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours