Bond characteristics
(1) The Bond length
The bond length is described as the average distance between the centres of nuclei of the two bonded atoms is generally termed as bond length.
It is expressed in the terms of Angstrom (1 Å = 10-10m) or picometer (1pm = 10-12m).
In the ionic compound, the bond length is the total sum of their ionic radii (d = r+ + r- ) and in the covalent compound, it is the sum of their covalent radii (such as for HCl, d= rH + rCl).
Factors affecting bond length
(i) The bond length gets increased with the increase in the size of the atoms. For example, bond length of H-X are in the order, HI > HBr > HCl > HF.
(ii) The bond length decreases with the multiplicity of the bond. Hence, bond length of the carbon-carbon bonds are in the order, 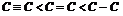 .
.
(iii) As the s-orbital is smaller in the size, greater the s-character shorter is the hybrid orbital and hence shorter is the bond length.
For instance, 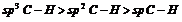
(iv) Polar bond length is generally smaller than the theoretical non-polar bond length.
(2) Bond energy
The amount of energy needed to break one mole of the bonds of a particular type so as to separate them into the gaseous atoms is known as bond dissociation energy or simply the bond energy. The Greater is the bond energy, the stronger is the bond. Bond energy is generally expressed in KJ Mol-1.
Factors affecting bond energy
(i) The Greater size of the atom, greater is the bond length and less will be the bond dissociation energy which means less is the bond strength.
(ii) For bond between the two similar atoms, greater is multiplicity of the bond, greater will be the bond dissociation energy.
(iii) The Greater the number of the lone pairs of electrons present on the bonded atoms, the greater will be the repulsion between the atoms and thus less is the bond dissociation energy.
(iv) The bond energy increases as hybrid orbitals have greater amount of s orbital contribution. Therefore, bond energy decreases in the following order, sp>sp2>sp3.
(v) The Greater electronegativity difference, the greater is the bond polarity and thus greater will be the bond strength that is bond energy, H-F>H-Cl>H-Br>H-I.
(vi) Among the halogens Cl - Cl > F - F > Br - Br > I - I, (Decreasing order of the bond energy) Resonance increases the bond energy.
(3) Bond angle
In case of the molecules made up of three or more than three atoms, the average angle between the bonded orbitals (that is., between the two covalent bonds) is called as bond angle θ.
Factors affecting bond angle
(i) The Repulsion between atoms or the groups attached to the central atom might increase or decrease the bond angle.
(ii) In the hybridisation as the s character of the s hybrid bond increases, bond angle increases.
|
Bond type
|
sp3
|
sp2
|
sp
|
|
Bond angle
|
109º28¢
|
120°
|
180°
|
(iii) By increasing the lone pair of electron, the bond angle decreases approximately by 2.5%.
|
|
CH4
|
NH3
|
H2O
|
|
Bond angle
|
109º
|
107o
|
105o
|
(iv) If the electronegativity of central atom decreases, the bond angle decreases.
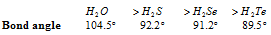
In case the central atom remains same, bond angle increases with decrease in electronegativity of the surrounding atom.
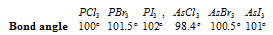
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Bond characteristics questions? Bond characteristics topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Bond characteristics related problems. We provide step by step Bond characteristics question's answers with 100% plagiarism free content. We prepare quality content and notes for Bond characteristics topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours