Bohr's atomic model
Bohr retained the essential features of the Rutherford model of an atom. Though, in order to account the stability of the atom he introduced the concept of the stationary orbits. The postulates Bohr are given as follows,
(1) An atom has a positively charged nucleus responsible for almost the entire mass of the atom (This assumption is retention of Rutherford model).
(2) The electrons are revolving around the nucleus in certain permitted circular orbits of definite radii.
(3) The permitted orbits are those for which the angular momentum of an electron is an integral multiple of 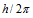 where h is Planck's constant. If m is the mass and v is the velocity of the electron in a permitted orbit of radius r then
where h is Planck's constant. If m is the mass and v is the velocity of the electron in a permitted orbit of radius r then
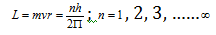
Where L is the orbital angular momentum and n is the number of orbit. The integer n is called the principal quantum number. This equation is generally known as the Bohr quantization postulate.
(4) When electrons move in permitted discrete orbits they do not radiate or lose any energy. Such type of orbits is called stationary or the non-radiating orbits. In this way, Bohr overcame Rutherford's difficulty to account for the stability of an atom. Greater the distance of the energy level from the nucleus, the larger is the energy related with it. The different energy levels were numbered as 1,2,3,4 .. and called as k, L, M, N, .... etc.
(5) Ordinarily the electron continues to move in the definate stationary state or orbit. Such type of state of an atom is called ground state. When the energy is given to the electron it jumps to any of the higher energy level and is said to be in excited state. When the electron jumps from the higher to lower energy state, the energy is radiated from it.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Bohr’s atomic model questions? Bohr’s atomic model topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Bohr’s atomic model related problems. We provide step by step Bohr’s atomic model question's answers with 100% plagiarism free content. We prepare quality content and notes for Bohr’s atomic model topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours