Autoxidation
(1) Turpentine and numerous other olefinic compounds, phosphorus and several metals such as Zn and Pb can absorb oxygen from air in the presence of water. The water is oxidised to the hydrogen peroxide. This phenomenon of the formation of H2O2 by oxidation of H2O is called as autoxidation. The substance like turpentine or phosphorus or lead which can activate the oxygen is termed as activator. The activator is supposed to first combine with the oxygen to form an addition compound, this act as the autoxidator and reacts with water or some other acceptor so as to oxidise the final. The example is given as follows
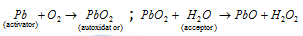
(2) The turpentine or the other unsaturated compounds which act as the activators are supposed to take up oxygen molecule at double bond position to form the unstable peroxide called moloxide, which then gives up oxygen to the water molecule or any other acceptor.
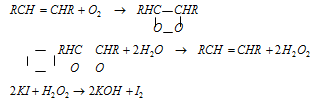
Evolution of the iodine from KI solution in presence of turpentine can be confirmed with starch solution which turns blue.
(3) The concept of autoxidation helps to explain the phenomenon of induced oxidation. Na2SO3 solution is oxidised by air but Na3AsO3 solution is not oxidised by air. If the mixture of both is taken, it is seen that both are oxidised. This is called as induced oxidation.
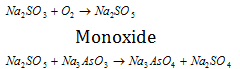
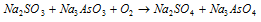
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Autoxidation questions? Autoxidation topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Autoxidation related problems. We provide step by step Autoxidation question's answers with 100% plagiarism free content. We prepare quality content and notes for Autoxidation topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours