Arrhenius concept : In accordance to Arrhenius concept all substances which give H+ ions when dissolved in water are called acids while those which ionise in water to furnish OH- ions are called bases.
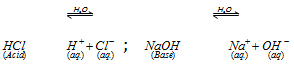
Some acids and bases ionise almost completely in solutions and are called as strong acids and strong bases. The others are dissociated to the limited extent in solutions and are termed weak acids and bases. , etc., are examples of strong acids and NaOH, KOH, (CH3)4NOH are strong bases. Each and every hydrogen compound cannot be regarded as an acid, for example CH4 is not an acid. Similarly, CH3OH, C2H5OH, etc., have OH groups but they are not bases.
(i) Utility of the Arrhenius concept : The Arrhenius concept of acids and bases was able to explain a number of phenomenon like neutralization, salt hydrolysis, strength of acids and bases etc.
(ii) Limitations of Arrhenius concept
(a) For the acidic or basic properties, presence of the water is quite necessary. Dry HCl shall not act as an acid. Hcl is regarded as an acid only when dissolved in water and not in any other solvent.
(b) The concept does not explain acidic and basic character of substances in non-aqueous solvents.
(c) The process neutralisation is limited to those reactions which can occur in the aqueous solutions only, although reactions involving salt formation do occur in absence of solvent.
(d) It cannot describe the acidic character of some specific salts such as in aqueous solution.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Arrhenius concept questions? Arrhenius concept topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Arrhenius concept related problems. We provide step by step Arrhenius concept question's answers with 100% plagiarism free content. We prepare quality content and notes for Arrhenius concept topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours