The Applications of solubility product
(i) In predicting formation of the precipitate
Case I : When Kip<Ksp, then solution is unsaturated in which more solute can be dissolved that is no precipitation.
Case II : When Kip=Ksp, then solution is saturated in which no more solute can be dissolved but no precipitate is formed.
Case III : When Kip>Ksp, then the solution is supersaturated and precipitation takes place.
When the ionic product exceeds solubility product, then the equilibrium shifts towards left-hand side that is increasing the concentration of undissociated molecules of the electrolyte. As solvent can hold the fixed amount of electrolyte at a specific temperature, the excess of the electrolyte is thrown out from the solutions as precipitate.
(ii) In predicting the solubility of the sparingly soluble salts knowing that the solubility product of a sparingly soluble salt at any specific temperature, we can predict its solubility.
(iii) Purification of common salt : HCl gas is circulated through the saturated solution of common salt. HCl and NaCl dissociate into their respective ions as,
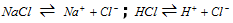
The concentration of Cl- ions increases considerably in solution due to ionisation of HCl and due to common ion effect, dissociation of NaCl is decreased. Therefore, the ionic product [Na+][Cl-] exceeds the solubility product of NaCl and therefore pure NaCl precipitates out from the solution.
(iv) Salting out of the soap : From solution, soap is precipitated when we add concentrated solution of NaCl.
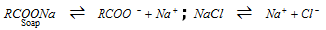
Therefore, the ionic product [RCOO-] [Na+] exceeds solubility product of soap and thus, soap precipitates out from the solution.
(v) In the qualitative analysis: The separation and the identification of several basic radicals into different groups are based upon solubility product principle and the common ion effect.
(a) Precipitation of the radicals of group first (Pb+2, Ag+ , Hg+2) The group reagent is dilute HCl. [Ag+][Cl-]> Ksp for AgCl.
(b) Precipitation of group second radicals (Hg+2, Pb+2, Bi+3, Cu+2, Cd+2, As+3, Sb+3 and Sn+2) : The group reagent is H2S in presence of dilute HCl. [Pb2+][S-2]>Ksp for PbS.
(c) Precipitation of group third radicals (Fe+3, Al+3 and Cr+3) The group reagent is NH4OH in presence of NH4Cl. [Fe+3][OH-]> Ksp
(d) Precipitation of group fourth radicals (Co+2, Ni+2, Mn+2 and Zn+2) : The group reagent is H2S in presence of NH4OH.
[Co2+][S-2]>Ksp
(e) Precipitation of group fifth radicals (Ba+2, Sr+2, Ca+2) The group reagent is ammonium carbonate in presence of NH4Cl and NH4OH.
[Ba2+][Co3-2]>Ksp
(vi) Calculation of remaining concentration after precipitation : Sometimes an ion remains after precipitation if it is in excess. The remaining concentration can be determined as follows,
Example : 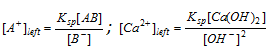 ;
;
In general 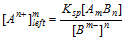
% precipitation of ion = 
(vii) Calculation of the simultaneous solubility : The solubility of two electrolytes possesing common ion; when they are dissolved in same solution, is known as simultaneous solubility.
Calculation of simultaneous solubility is divided into two cases.
Case I : When two electrolytes are nearly equally strong (having close solubility product).
e.g., 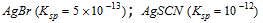 ;
;
The charge balancing concept is applied hear.
Charge of Ag+ = Charge of Br- + Charge of SCN-
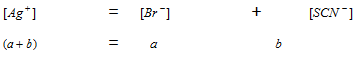
Case II : When the solubility products of the two electrolytes are not close, which means they are not equally strong.
e.g., 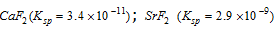 ;
;
Most of the fluoride ions come of the stronger electrolyte.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Applications of solubility product questions? Applications of solubility product topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Applications of solubility product related problems. We provide step by step Applications of solubility product question's answers with 100% plagiarism free content. We prepare quality content and notes for Applications of solubility product topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours