Applications of Inductive effect
(i) Magnitude of negative and positive charges : Magnitude of +ve charge on cations and magnitude of -ve charge on anions can be compared by + I or - I groups present in it.
· Magnitude of +ve charge 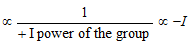 power of the group.
power of the group.
· Magnitude of -ve charge 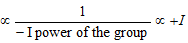 power of the group.
power of the group.
(ii) Reactivity of alkyl halide : + I effect of methyl group enhances - I effect of the halogen atom by repelling the electron towards tertiary carbon atom.
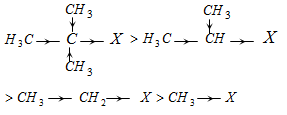
Tertiary > Secondary > Primary > Methyl
(iii) Relative strength of the acids :
(a) Any group or atom showing +I effect decreases the acid strength as it increases the negative charge on the carboxylate ion which holds the hydrogen firmly. Alkyl groups have + I effect.
Thus, acidic nature is,
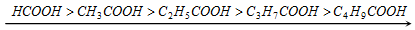
+I effect increases, so acid strength decreases
Formic acid, obtain no alkyl set, is the most acidic among these acids.
(b) The group or atom having - I effect increases the acid strength as it decreases the negative charge on the carboxylate ion. Greater is the number of such molecules or sets (having - I effect), greater is the acid strength.
Thus, acidic nature is,
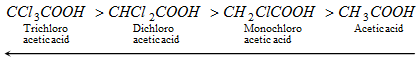
(- Inductive effect added, so acid power increases)
(c) Strength of benzoic acids and aliphatic carboxylic acid
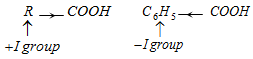
Hence benzoic acid is stronger acid than aliphatic carboxylic acids but exception is formic acid. So,
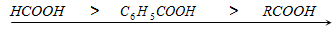
Acid strength in decreasing order
- Decreasing order of acids :
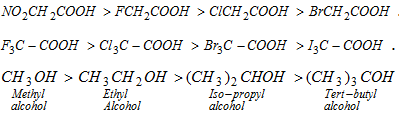
As compared to water, phenol is more acidic (-I effect) but methyl alcohol is less acidic (+I effect).
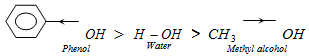
(vi) Relative strength of the bases (Basic nature of -NH2)
The difference in base strength in various amines can be explained on the basis of inductive effect. The +I effect increases the electron density while -I effect decreases it. The amines are stronger bases than NH3 as the alkyl groups increase electron density on nitrogen due to + I effect while CINH2 is less basic due to -I effect. "So more is the tendency to donate electron pair for manage with proton, the more is basic function, i.e., more is the negative charge on nitrogen molecule (due to +I effect of alkyl group), the more is base nature".
Thus, the basic nature decreases in the order;
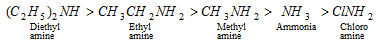
The order of basicity is as shown in table;
|
Alkyl groups (R- )
|
Relative base strength
|
|
CH3
|
R2NH > RNH2 > R3N > NH3
|
|
C2H5
|
R2NH > RNH2 > NH3 > R3N
|
|
(CH3)2CH
|
RNH2 > NH3 > R2NH > R3N
|
|
(CH3)3C
|
NH3 > RNH2 > R2NH > R3N
|
- The relative basic character of amines is not in total accordance with inductive effect (t > s > p) but it is in the following order: Secondary > Primary > Tertiary. The reason is the steric hindrance existing in the t-amines.
- In gas phase or in aqueous solvents such as chlorobenzene etc, the solvation effect, i.e., the organization of the conjugate acid due to H-bonding are absent and hence in these media the basicity of amines depends only on the +I effect of the alkyl group thus the basicity of amines follows the order : 30 > 20 > 10 > NH3.
(vii) Basicity of alcohols : The decreasing order of base strength in alcohols is due to +I effect of alkyl groups.
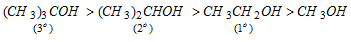
(viii) Stability of carbonium ion :+I effect tends to decrease the (+ve) charge and -I effect tends to increases the +ve charge on carbocation.
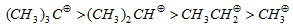
(ix) Stability of carbanion : Stability of carbanion increases with increasing - I effect.
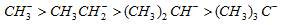
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Applications of Inductive effect questions? Applications of Inductive effect topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Applications of Inductive effect related problems. We provide step by step Applications of Inductive effect question's answers with 100% plagiarism free content. We prepare quality content and notes for Applications of Inductive effect topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours