Applications of hyperconjugation
(1) Stability of alkenes : Hyperconjugation explains the stability of certain alkenes over other alkenes.
Stability of alkenes ∝ Number of alpha hydrogens ∝ Number of resonating structures
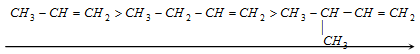
Stability in decreasing order
(2) Carbon-carbon double bond size in alkenes : As we know that the more is the number of resonating structures, the more will be single bond character in carbon-carbon double bond.
(3) Durability of alkyl carbocations : Stability of alkyl carbocations ∝ number of resonating structures µ number of alpha hydrogens.
(4) Stability of alkyl free radicals : Stability of alkyl free radicals can be explained by hyperconjugation. Stability relays on the number of resonating structures.
(5) Electron releasing (or donating) power of R in alkyl benzene : CH3- (or alkyl group) is +R group, ortho-para directing set and activating set for electrophilic aromatic substitution reaction because of the hyperconjugation.
The electron donating power of alkyl group will depends on the number of resonating structures, this relays on the number of hydrogens present on a-carbon. The electron releasing power of some groups are as follows,
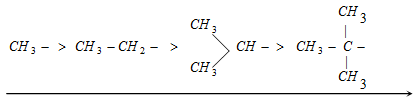
Increasing inductive effect
Electron donating power in descending order due to the hyperconjugation.
(6) Heat of hydrogenation : Hyperconjugation decreases the heat of hydrogenation.
(7) Dipole moment : Since hyperconjugation affects the development of charges, it also affects the dipole moment in the molecule.
The increase in dipole moment, when hydrogen of formaldehyde (μ = 2.72D) is replaced by methyl group, i.e., acetaldehyde (μ = 2.72D) can be tends to hyperconjugation, which tends to development of charges.
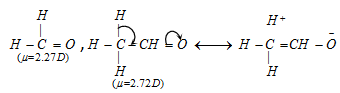
(8) Orienting influence of alkyl group in o,p-positions and of -CCl3 group in m-position : Ortho-para directing property of methyl group in toluene is partly due to effect and partly due to hyperconjugation.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Applications of hyperconjugation questions? Applications of hyperconjugation topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Applications of hyperconjugation related problems. We provide step by step Applications of hyperconjugation question's answers with 100% plagiarism free content. We prepare quality content and notes for Applications of hyperconjugation topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours