Applications of hybridisation
(i) Size of the hybrid orbitals : Since s- orbitals are closer to the nucleus than p- orbitals, it is reasonable to expect that greater the s character of an orbital the smaller it is. Thus the descending order of the length of the three hybrid orbitals is opposite to that of the decreasing order of s orbital character in the three hybrid orbitals.
sp3 > sp2>sp
(ii) Electronegativity of different orbitals
(a) Electronegativity of s-orbital is highest.
(b) Electronegativity of hybrid orbital ∝ % s-character in hybrid orbitals
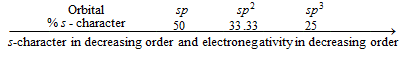
Thus sp-hybrid carbon is always electronegative in character and sp3- hybrid carbon is electropositive in character. Sp2-hybrid carbon can behave as electropositive (in carbocation) as well as electronegative (in carbanion) in character.
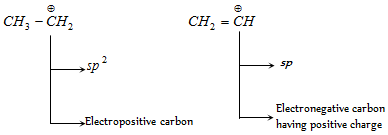
(c) Electronegativities of distinct hybrid and unhybrid orbitals in decreasing order is as follows
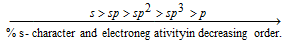
(iii) Bond length variation in hydrocarbons
% of s orbital character
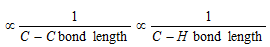
Table :
|
Bond type (C - H)
|
Bond length
|
Bond type (C - C)
|
Bond length
|
|
sp3 - s (alkanes)
|
1.112Å
|
sp3 - sp3 (alkanes)
|
1.54 Å
|
|
sp2 - s (alkenes)
|
1.103Å
|
sp2 - sp2 (alkenes)
|
1.34Å
|
|
sp - s (alkynes)
|
1.08Å
|
sp - sp (alkynes)
|
1.20Å
|
(iv) Bond strength in hydrocarbons : The shorter is the bond size, the greater is the compression between molecules atomic nuclei and hence greater is the strength of that bond.
Table :
|
Bond type (C - H)
|
Bond energy
(kcal/mole)
|
Bond type (C - C)
|
Bond energy
(kcal/mole)
|
|
sp3 - s
(in alkanes)
|
104
|
sp3 - sp3
(in alkanes)
|
80 - 90
|
|
sp2 - s
(in alkenes)
|
106
|
sp2 - sp2
(in alkenes)
|
122 - 164
|
|
sp - s
(in alkynes)
|
121
|
sp - sp
(in alkynes)
|
123 - 199
|
(v) Acidity of hydrocarbons
(a) Hydrogen exist on electronegative carbon is acidic in nature.
(b) Acidity of hydrogen is directly proportional to the electronegativity of the atom on which hydrogen is present.
Thus

(c) Acidity of hydrocarbon ∝ % of s-character
% s-character 50 33.33 25
25 44 50
s- character and acidity in descending order
Acidity 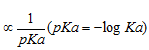
Order of acidic nature of alkynes is,
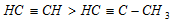
The relative acidic character takes the order;
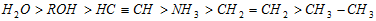
Obviously, the basic character of their conjugate bases follows the reverse sequence, i.e.,
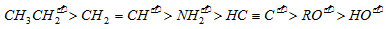
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Applications of hybridisation questions? Applications of hybridisation topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Applications of hybridisation related problems. We provide step by step Applications of hybridisation question's answers with 100% plagiarism free content. We prepare quality content and notes for Applications of hybridisation topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours