Application of radioactivity
Radioisotopes find number of applications in several of areas such as medicine, engineering biology, chemistry, archeology, agriculture, and industry.
(1) Age determination : The age of earth has been determined by uranium dating methods as follows. Samples of uranium ores are found to contain Pb206 as a result of long series of α- and β-decays. Now if it is assumed that the ore sample contained no lead at the moment of its formation, and if none of the lead formed from U238 decay has been lost then the measurement of the Pb206/ U238 ratio will give the value of time t of the mineral.
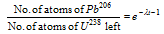
here λ is the decay constant of the uranium-238
Alternatively,
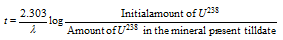
Similarly, the less abundant isotope of uranium, U235 eventually decays to Pb207 ; Th232 decays to Pb208 and thus the ratios of Pb207/ U235 and Pb208/ Th232 can be used to determine the age of rocks and minerals.
6C14 (half-life 5760 years) was used by Willard Libby (Nobel lauret) in determining the age of carbon-bearing materials (such as animal fossils, wood, etc.) Carbon-14 is produced by the bombardment of nitrogen atoms present in the upper atmosphere with neutrons (from cosmic rays).
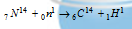
Thus carbon-14 is oxidised to CO2 and eventually ingested by plants and animals. The death of the plants and animals puts an end to the intake of C14 from the atmosphere. After this the amount of C14 in the dead tissues starts decreasing due to its disintegration.
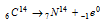
It has been observed that in average, one gram of the radioactive carbon emits about 12 β -particles per minute. Hence by knowing either the amount of C-14 or the number of β -particles emitted per minute per gram of carbon at the initial and final (like present) stages, age of carbon material can be determined by using the following formulae.
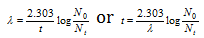
or
here t = Age of the fossil, λ = Decay constant, N0= Initial radioactivity (in the fresh wood), Nt= Radioactivity in the fossil
The above formula can be modified as,
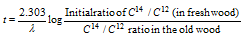
Similarly, tritium 1H3 has been used for dating purposes.
(2) Radioactive tracers (use of radio-isotopes) : A radioactive isotope can be simply identified by its radioactivity. The radioactivity can, thus act as a tag or label that allows studying the behavior of the element or compounding which contains this isotope. An isotope added for this purpose is known as isotopic tracer. The radioactive tracer is also called as an isotopic tracer. The radioactive tracer is called as an indicator because it indicates reaction. The radioisotopes of moderate half-life periods are used for tracer work. The activity of the radioisotopes can be detected by means of the electroscope, electrometer or Geiger-Muller counter. Tracers have been used in the fields as discussed below,
(i) To diagnose many diseases : For instance, Arsenic - 74 tracer is used to detect presence of tumours, Sodium - 24 tracer is used to detect presence of blood clots and Iodine -131 tracer is used to study activity of the thyroid gland. It should be noted that radioactive isotopes used in medicine have very short half-life periods.
(ii) In agriculture : Use of the radioactive phosphorus 32P in the fertilizers has revealed how phosphorus is absorbed by the plants. This study has led to a development in the preparation of fertilizers. 14C is used to study kinetics of photo synthesis.
(iii) In industry : The radioisotopes are taken in use in industry to detect leakage in the underground oil pipelines, gas pipelines and the water pipes. Radioactive carbon has been taken in use as a tracer in studying mechanisms involved in number of reactions of industrial importance such as polymerization, catalytic synthesis alkylation and many more.
(iv) In analysis: Number of analytical procedures can be used as employing radioisotopes as tracers.
(a) A small quantity of the radioactive isotope is mixed with the inactive substance and activity is studied before and after the adsorption. Fall in activity provides the amount of the substance adsorbed.
(b) The solubility of lead sulphate in water can be estimated by mixing the known amount of radioactive lead with an ordinary lead.
(c) Ion exchange process of the separation is readily followed by the measuring activity of successive fractions eluted from column.
(d) By labelling oxygen of water, mechanism of the ester hydrolysis has been studied.
(e) The efficiency of the analytical procedures can be measured by adding a known amount of the radio-isotopes to sample before the analysis starts. After the completion, activity is again determined. The comparison of the activity tells us about the efficiency of the separation.
(3) Use of γ rays : γ rays are taken in use for disinfecting food grains and for preserving the food stuffs. Onions, fruits potatoes, and fish and many more when irradiated with γ rays, can be preserved for the long periods. The high yielding disease resistant varieties of the groundnut, jute, wheat, rice, etc., can be developed by application of the nuclear radiations. The γ rays radiations are used in the treatment of cancer. The γ radiations emitted by the cobalt -60 can burn cancerous cells. The γ radiations are used to sterilize the medical instruments such as blood transfusion sets syringes etc. These radiations make rubber and plastics objects heat resistant.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Application of radioactivity questions? Application of radioactivity topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Application of radioactivity related problems. We provide step by step Application of radioactivity question's answers with 100% plagiarism free content. We prepare quality content and notes for Application of radioactivity topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours