Anomalous behaviour of Lithium
Anomalous behaviour of lithium is due to extremely small size of lithium its cation on account of small size and high nuclear charge, lithium exerts greatest polarizing effect out of all the alkali metals on the negative ion. As a result lithium ion have remarkable tendency towards the solvation and develops the covalent character in the compounds of it. Li differs from other alkali metals in the below stated respects,
(1) It is comparatively harder than the other alkali metals. Li can'nt be stored in the kerosene as it floats to surface, because of its very low density. Li is commonly kept wrapped in the parrafin wax.
(2) It can be melted in the dry air without losing its brilliance.
(3) Not like other alkali metals, it is least reactive among all. It can be noticed by the properties stated below,
(i) It is not affected by air.
(ii) It decomposes water slowly to liberate H2.
(iii) It hardly reacts with the bromine while other alkali metals react violently with it.
(4) Lithium is only alkali metal which directly reacts with the N2 to form Lithium nitride (Li3N)
(5) Lithium when heated in the NH3 forms amide, Li2 NH while other metals form the amides, which is MNH2.
(6) When burnt in the air, lithium form Li2O sodium form Na2O and Na2O2 other alkali metals form the monoxide, peroxide and superoxide.
(7) Li2O is much less basic and less soluble in water than the other alkali metals.
(8) LiOH is weaker base than the NaOH or KOH and decomposes while heating.
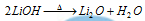
(9) LiHCO3 is liquid in nature while other metal bicarbonates are solid.
(10) Only Li2CO3 decomposes on heating which is shown below
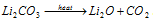
Na2CO3, K2CO3 etc. do not decompose on heating.
(11) LiNO3 and other alkali metal nitrates provides different products on heating
4LiNO3 = 2Li2O+4NO2 + O2 ; 2NaNO3 = 2NaNO2 + O2
(12) LiCl and LiNO3 are soluble in the alcohol and other organic solvents. These salts of the other alkali metals are, though, insoluble in organic solvents.
(13) LiCl is deliquescent while the NaCl, KBr etc. are not. Lithium chloride crystals comprise two molecules of the water of crystallisation (LiCl. 2H2O). Crystals of NaCl KBr, KI etc do not conation water of the crystallisation.
(14) Li2SO4 does not form alums as other alkali metals.
(15) Li reacts with the water slowly at room temperature Na reacts vigorously Reaction with the K Rb and Cs is quite violent.
(16) Li reacts with Br2 slowly. Reaction of the other alkali metals with Br2 is fast.
(17) Li2 CO3 Li2C2O4, LiF , Li3PO4 are the only alkali metal salts which are insoluble or sparingly soluble in water.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Anomalous behaviour of Lithium questions? Anomalous behaviour of Lithium topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Anomalous behaviour of Lithium related problems. We provide step by step Anomalous behaviour of Lithium question's answers with 100% plagiarism free content. We prepare quality content and notes for Anomalous behaviour of Lithium topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours