The analysis of the cubic system
(1) The number of atoms in per unit cell
Total number of atoms enclosed in the unit cell for the simple cubic called as unit cell content.
The simplest relation which can be determine for it is, 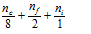
Here nc = Number of the atoms at corners of the cube=8
nf = Number of the atoms at six the faces of the cube = 6
ni = Number of the atoms inside the cube = 1
Cubic unit cell
|
nc
|
nf
|
ni
|
Total atom in per unit cell
|
|
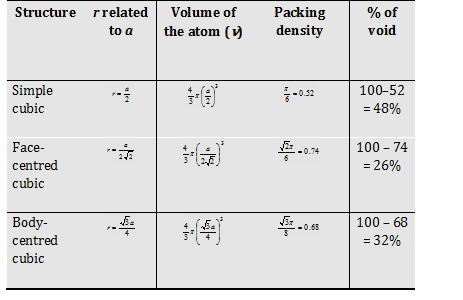
Simple cubic (sc)
|
8
|
0
|
0
|
1
|
|
body centered cubic (bcc)
|
8
|
0
|
1
|
2
|
|
Face centered cubic (fcc)
|
8
|
6
|
0
|
4
|
(2) Co-ordination number (C.N.) : The co-ordinate number is defined as the number of the nearest neighbours or touching particles with other particle present in the crystal is known as its co-ordination number. It depends upon the structure of the crystal.
For simple cubic system Cubic number = 6.
For body centred cubic system Cubic number = 8
For face centred cubic system Cubic number = 12.
(3) Density of the unit cell 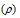 : It can be defined as the ratio of mass per unit cell to the total volume of the unit cell.
: It can be defined as the ratio of mass per unit cell to the total volume of the unit cell.
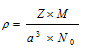
Where Z = Number of particles per unit cell
M = Atomic mass or molecular mass
No = Avogadro number (6.023 * 1023 mol-1)
a = Edge length of the unit cell= a pm = a * 10-10 cm
a3 = volume of the unit cell
i.e. 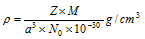
The density of substance is same as the density of the unit cell.
(4) Packing fraction (P.F.): It can be defined as ratio of the volume of the unit cell that is occupied by spheres of the unit cell to the total volume of the unit cell.
Let radius of the atom in the packing = r
Edge length of the cube = a
Volume of the cube V = a3
Volume of the atom (spherical) = 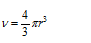
Packing density 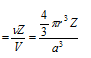
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Analysis of Cubic System questions? Analysis of Cubic System topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Analysis of Cubic System related problems. We provide step by step Analysis of Cubic System question's answers with 100% plagiarism free content. We prepare quality content and notes for Analysis of Cubic System topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours