Alkali Metals and Their Compounds
The group 1 of the periodic table contains six elements, namely lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr). All such elements are typical metals. The francium is radioactive with the longest lived isotope 223Fr with half life period of only 21 minute. These are generally referred to as alkali metals since their hydroxides form the strong bases or alkalies.
(1) Electronic configuration
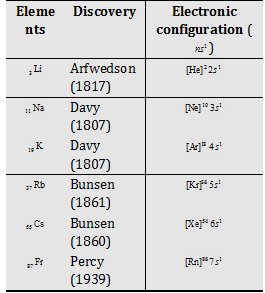
(2) Occurrence : Alkali metals are very reactive and thus found in combined state some important ores of alkali metals are given ahead.
(i) Lithium : Triphylite, Petalite, lepidolite, Spodumene [LiAl(SiO3)3], Amblygonite [Li(Al F)PO4]
(ii) Sodium : Chile salt petre (NaNO3), Sodium chloride (NaCl), Sodium sulphate (Na2SO4), Borax (Na2B4O710H2O), Glauber salt (Na2 SO4.10H2O)
(iii) Potassium : Sylime (KCl), carnallite (KCl.MgCl2.6H2O) and Felspar whose chemical formula is(K2O.Al2O3.6SiO2)
(iv) Rubidium : Lithium ores Lepidolite, triphylite contains 0.7 to 3% Rb2O
(v) Caesium : Lepidolite, Pollucite contains 0.2 to 7% Cs2O
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Alkali metals questions? Alkali metals topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Alkali metals related problems. We provide step by step Alkali metals question's answers with 100% plagiarism free content. We prepare quality content and notes for Alkali metals topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours