Advantages of Bohr's theory
(i) Bohr's theory satisfactorily explains the spectra of species having one electron, viz. hydrogen atom, He+, Li2+etc.
(ii) Calculation of radius of Bohr's orbit : According to Bohr, radius of nth orbit in which electron moves is
.png)
Where, n = Orbit number, m = Mass number (9.1 * 10-31 Kg), e = Charge on the electron (1.6 * 10-19) z = Atomic number of element, k = Coulombic constant 9 * 109Nm2c-2
After we put the values of m,e,k,h, we obtain.
.png)
(iii) Calculation of the velocity of an electron
.png)
(iv) Calculation of the energy of electron in the Bohr's orbit
Total energy of electron = K.E. + P.E. of electron .png)
Substituting of r, gives us .png) Where, n=1, 2, 3..........
Where, n=1, 2, 3..........
Putting the value of m, e, k, h,∏ we obtain
.png)
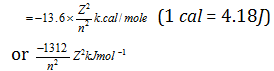
When the electron jumps from the outer orbit (which is higher energy) n2 to the inner orbit (which is lower energy) n1 then the energy is emitted in the form of radiation which is given by
.png)
As we know that .png) and
and .png)
.png)
This can be represented as .png)
Where, .png) ; R is known as Rydberg constant. Hear the value to be used is 109678 cm-1
; R is known as Rydberg constant. Hear the value to be used is 109678 cm-1
The negative sign in above equations signifies that the electron and the nucleus form a bound system, which means that the electron is attracted towards the nucleus. Hence, if electron is to be taken away from nucleus, energy has to be supplied. The energy of the electron in n = 1 orbit is known the ground state energy; that in the n = 2 orbit is called the first excited state
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Advantages of Bohr’s theory questions? Advantages of Bohr’s theory topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Advantages of Bohr’s theory related problems. We provide step by step Advantages of Bohr’s theory question's answers with 100% plagiarism free content. We prepare quality content and notes for Advantages of Bohr’s theory topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours