Activity and Selectivity
(1) Activity : Activity is the ability of catalysts to accelerate chemical reaction, degree of the acceleration can be as high as 1010 times in certain reactions. For instance reaction between H2 and O2 to form H2O in presence of platinum as the catalyst takes place with explosive violence.
In absence of catalyst, H2 and O2 can be stored indefinitely without any reaction.
(2) Selectivity : This the ability of catalysts to direct reaction to yield particular products (excluding other).
Example :
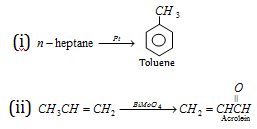
Zeolite (Shape selective catalysis)
(1) Zeolite are alumino-silicates of the general formula, Mx/n[AlO2]x.(SiO2)y.mH2O, where, M may be simple cation such as Na+, K+ or Ca2+, n is the charge on the simple cation, m is number of molecules of water of crystallization.
(2) Some of the well known zeolites are as follows,
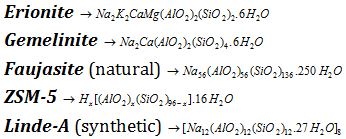
(3) The characteristics of zeolites are the openness of the structure, which allows cavities of different sizes.
(4) The open structure is provided by silica in which aluminium occupies x/(x+y) fraction of the telrahedral sites.
(5) Negative charge of the aluminosilicate framework can be neutralized by the replaceable cations.
(6) The void space forms more than 50% of the total volume, which is occupied by water molecules.
(7) The reaction- selectivity of zeolites depends upon the size of cavities (cages), pores (apertures) and the distribution of pores in the structure. The pore size in the zeolites usually varies from 260 pm to 740 pm.
(8) Zeolite has high porosity due to the presence of may be one, two, or three dimensional networks of the interconnected channels and cavities of the molecular dimensions.
(9) There is a new class of highly siliceous zeolites with an optimal pore diameter of 550pm. The ZSM-5 is one such zeolite having the formula. [Hx(AlO2)x.SiO2)96-x].16H2O
(10) The zeolite catalyst which is ZSM-5 converts alcohols to gasoline (petrol) by dehydrating alcohol and producing the mixture of wide variety of hydrocarbons.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Activity and Selectivity questions? Activity and Selectivity topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Activity and Selectivity related problems. We provide step by step Activity and Selectivity question's answers with 100% plagiarism free content. We prepare quality content and notes for Activity and Selectivity topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours