Acidity of Aromatic Carboxylic Acid : Aromatic acid dissociates to give a carboxylate anion and proton.
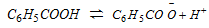
Since the carboxylate anion  is resonance stabilised to a greater extent than the carboxylic acid (ArCOOH).
is resonance stabilised to a greater extent than the carboxylic acid (ArCOOH).
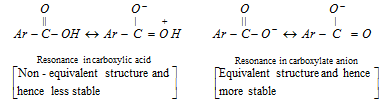
Effect of Substituents on Acidity : The overall influence of a substituent on acidity of substituted benzoic acids is due to two factors.
(i) Inductive effect : If the substituent exerts-I effect, it increases the acidity of carboxylic acids, while if it exerts + I effect it decreases the acidity. Inductive effect affects all positions, i.e., o-, m- and p-.
(ii) Resonance effect : Like inductive effect, if the resonance producing group exerts minus effect i.e., if it withdraws electrons, it increases the strength of the benzoic acid. Similarly, if the group causes +R affect it decreases the acidity of benzoic acid. However, remember that resonance effect affects only o- and p- positions. Thus if resonance producing group is present in the m-position it will not exert its effect.
In case resonance and inductive effects both operate in the molecule, resonance effect being stronger overpowers the inductive effect.
Thus on the above basis, the following order of acidity can be explained.
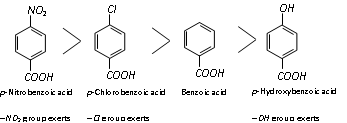
Similarly :
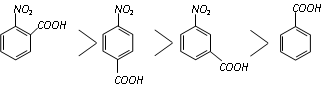
Acidity is only because of electron withdrawing inductive effect of the - NO2 group (resonance does not affect the m-position) while in the p-isomer acidity is due to electron withdrawing inductive as well as resonance effect.
The acidity of the three isomers of hydroxybenzoic acids follows the following order.
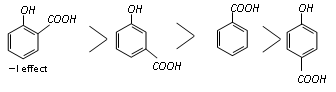
Resonance effect cannot operate and hence only the acid-strengthening -I effect takes part with the result m-hydroxybenzoic acid is stronger acid than benzoic acid. Like other substituted benzoic acid.
Acidic character surrounded by benzoic acids having different electron releasing group.
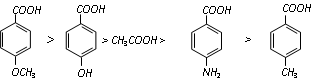
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Acidity of Aromatic Carboxylic Acid questions? Acidity of Aromatic Carboxylic Acid topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Acidity of Aromatic Carboxylic Acid related problems. We provide step by step Acidity of Aromatic Carboxylic Acid question's answers with 100% plagiarism free content. We prepare quality content and notes for Acidity of Aromatic Carboxylic Acid topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours