Acidic nature of monocarboxylic acids
(1) Cause of acidic nature
(i) A atom of carboxylic acid may be shown as a resonance hybrid of the following structures.
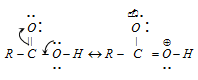
(I) (II)
(ii) Due to electron deficiency on oxygen atom of the hydroxyl group (Structure II), there is a distance of electron pair of O-H bond toward the oxygen molecule. These facilitate the release of hydrogen as proton (H+).
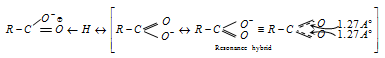
(iii) The resulting carboxylate ion also stabilized by resonance (As negative charge is dispersed on both the oxygen atom). It increases the stability of carboxylate anion and makes it weaker base or strong acid.
(2) Effect of substituent on acidic nature
(i) An electron withdrawing substituent (- I effect) stabilizes the anion by dispersing the negative charge and therefore increases the acidity.
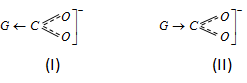
(ii) An electron releasing substituent (+ I effect) stabilizes negative charge on the anion resulting in the decrease of stability and thus decreased the acidity of acid.
Electron with drawing nature of halogen : F > Cl > Br > I
Thus, the acidic strength decreases in the order :
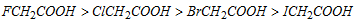
similarly :
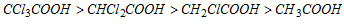
(iii) Inductive effect is stronger at a-position than b-position similarly at b-position it is more stronger than at  -position
-position
Example:
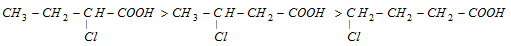
(iv) Relative acid strength in different compounds
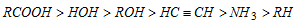
- Greater the value of Ka or lesser the value of pKa stronger is the acid, i.e. pKa=log Ka
- Acidic nature (Ka) a 1/molecular weight
-

- The formic acid is powerful of all fatty acids.
- Acetic acid is less weak acid than sulphuric acid due to less degree of ionisation.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Acidic nature of monocarboxylic acids questions? Acidic nature of monocarboxylic acids topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Acidic nature of monocarboxylic acids related problems. We provide step by step Acidic nature of monocarboxylic acids question's answers with 100% plagiarism free content. We prepare quality content and notes for Acidic nature of monocarboxylic acids topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours