The acid-base neutralisation and Salt
The reaction among an acid and a base to form salt and water is termed neutralisation
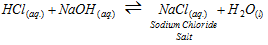
The process of neutralisation does not produce the resulting solution always neutral; no doubt it involves the interaction of H+ and OH- ions. The nature of the resulting solution depends on the particular acid and the particular base involved in the reaction.
Salts : The salts are regarded as compounds made up of positive and negative ions. The positive part comes from a base while negative part from an acid. Salts are ionic compounds. The salts can be classified into following classes,
(1) Simple salts : The salt formed by the interaction among acid and base, is called as simple salt. These are of three types,
(i) Normal salts : the salts formed by the loss of all possible protons (replaceable hydrogen atoms as H+) are called normal salts. This type of salt does not contain either replacable hydrogen or a hydroxyl group.
Examples : NaCl, NaNO3, K2SO4, Ca3(PO4)2, Na3BO3, Na2HPO3 (one H atom is not replaceable as H3PO2 is a dibasic acid) NaH2PO2 (both H atoms are not replaceable as H3PO2 is a monobasic acid) etc.
(ii) Acidic salts : Salts formed by incomplete neutralisation of poly-basic acids are called acidic salts. This type of salts still contain one or more replaceable hydrogen atoms. These salts when are neutralised by the bases form normal salts.
Examples : NaHCO3, NaHSO4, NaH2PO2, etc.
(iii) Basic salts : Salts formed by incomplete neutralisation of poly acidic bases are called basic salts. This type of salts still contains one or more hydroxyl groups. These salts when are neutralised by the acids form normal salts.
Examples: Zn(OH)Cl, Mg(OH)Cl, Fe(OH)2Cl, Bi(OH)2Cl
(2) Double salts : The addition compounds formed by combination of the two simple salts are termed as double salts. This type of salts are stable in solid state only.
Examples : Ferrous ammonium sulphate, Potash alum and other alums.
(3) Complex salts : These are formed by combination of simple salts or the molecular compounds. These molecular compounds are stable in solid state as well as in solutions.
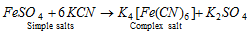
(4) The Mixed salts : The salt which furnishes more than one cation or more than one anion when dissolved in water is called a mixed salt.
Examples : 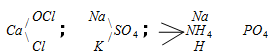
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Acid-base Neutralisation and Salt questions? Acid-base Neutralisation and Salt topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Acid-base Neutralisation and Salt related problems. We provide step by step Acid-base Neutralisation and Salt question's answers with 100% plagiarism free content. We prepare quality content and notes for Acid-base Neutralisation and Salt topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours