Acetic Acid (Ethanoic Acid) (CH3COOH)
Acetic acid is the oldest known fatty acid. It is the chief constituent of vinegar and hence its name (Latin acetum = vinegar)
(1) Preparation
(i) By oxidation of acetaldehyde (Laboratory-preparation)
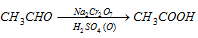
(ii) By hydrolysis of methyl cyanide with acid
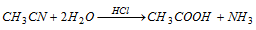
(iii) By Grignard reagent
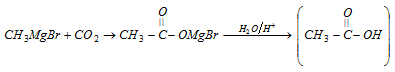
(iv) By hydrolysis of acetyl chloride, acetic anhydride or acetamide and ester
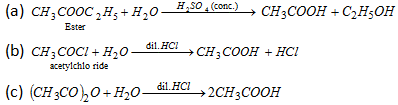
(v) Manufacture of acetic acid
(a) From ethyl alcohol (Quick vinegar process) : Vinegar is 6-10% aqueous solution of acetic acid. It is obtained by fermentation of liquors containing 12 to 15% ethyl alcohol. Fermentation is done by Bacterium Mycoderma aceti in presence of air at 30-35°C. The process is termed acetous fermentation.

It is a slow process and takes about 8 to 10 days for completion.
In this process, the following precautions are necessary:
· The concentration of the ethyl alcohol could not be more than 15%, otherwise the bacteria becomes inactive.
· The supply of air should be regulated. With less air the oxidation takes place only upto acetaldehyde stage while with excess of air, the acid is oxidised to CO2 and water.
· The flow of alcohol is so regulated that temperature does not exceed 35°C, which is the optimum temperature for bacterial growth.
Acetic acid can be obtained from vinegar with the help of lime. The calcium acetate crystallised from the solution is distilled with concentrated sulphuric acid when pure acetic acid distils over.
(b) By the action of CO on methyl alcohol : Methyl alcohol and carbon monoxide react together under a pressure of 30 atmospheres and 200°C in presence of a catalyst cobalt octacarbonyl, Co2(CO)8 to form acetic acid.
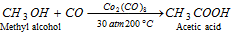
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Acetic Acid (Ethanoic Acid) questions? Acetic Acid (Ethanoic Acid) topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Acetic Acid (Ethanoic Acid) related problems. We provide step by step Acetic Acid (Ethanoic Acid) question's answers with 100% plagiarism free content. We prepare quality content and notes for Acetic Acid (Ethanoic Acid) topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours