Amphoteric Exchangers:
The ion exchangers that contain both acidic and basic groups are called as amphoteric exchangers. A number of exchangers of this type have been synthesized but only a few have found application.
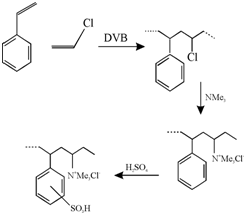
A well known resin holding both strong base and acid groups is prepared through copolymerization of styrene, vinylchloride and a cross-linking agent followed by quaternization and sulphonation of the product.
Among the amphoteric resins, the most significant are the ones known as snake- cage polyelectrolytes. That is conventional cation or anion exchangers within that polycation or polyanions, correspondingly have been created through polymerization. A typical example is that a snake- cage polyelectrolyte can be prepared by converting a strong base anion exchanger to acrylate form and then acrylate anion is polymerized in the resin. The linear chains of the poly-counter ions are so intricately interwined with the crosslinked matrix that they cannot be displaced by other counter ions. The situation is something like a snake trapped within a cage. One important difference these snake cage polyelectrolytes show from other amphoteric exchangers is that the poly-counter ions are not attached to the matrix. Thus, the charges of poly-counter ions of the matrix have more freedom to move. As a conclusion, it is not necessary for the resin to have mobile counter ions (counter ions to the poly-counter ions) to remain electrically neutral provided the charges of fixed ionic groups and poly-counter ions are balanced. These exchangers are excellent reversible sorbents for electrolytes. This will be discussed later when the applications of ion exchangers are being cited.
At the end of this section on the synthesis of ion exchange resins, it may be important to point out that the chemical structures of the polymers shown are hypothetical. It is difficult to establish the resin structure exactly. Furthermore, the structures of the polymers do not represent repeating identical units since the sequence of the monomeric component is essentially random.