Nitrile groups:
Exactly similar theory can be employed to describe the bonding in a nitrile group (C≡N) in which both the carbon and the nitrogen are sp hybridized. The energy level diagram in below diagram displays how the valence electrons of nitrogen are arranged after sp hybridization. A lone pair of electrons takes place one of the sp orbitals, but the other sp orbital can be employed for a strong σ bond. The 2py and 2pz orbitals can be employed for two π bonds. Below diagram presents the σ bonds of HCN as lines and how the remaining 2p orbitals are employed to form two π bonds.
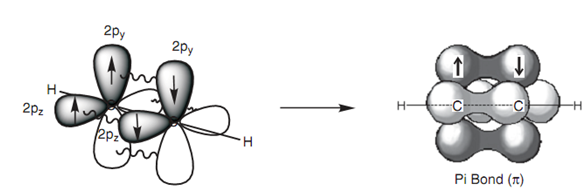
Figure: π-Bonding in ethyne.
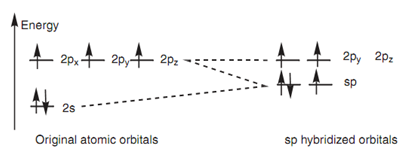
Figure: sp Hybridization of nitrogen
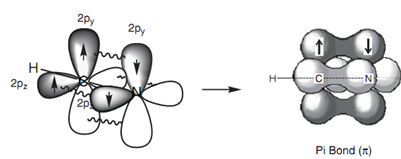
Figure: π-Bonding in HCN.