Zinc finger:
Various kinds of zinc finger have been reported, two of that are the C2H2 finger, the C4 finger. The C2H2 zinc finger is a loop of 12 amino acids with two cysteines and two histidines at the base of the loop that tetrahedrally coordinate a zinc ion in the figure. This forms a compact structure of two β-strands and one α- helix. The α-helix holds a number of conserved basic amino acids and interacts straightly with the DNA, binding in the main groove of the double helix. The Transcription factors that contain zinc fingers often contain several such motifs, arranged such that α-helix of every contacts the DNA. Certainly RNA polymerase III transcription factor A contains nine zinc fingers! The SP1 transcription factor that binds to the SP1 box has three zinc fingers.
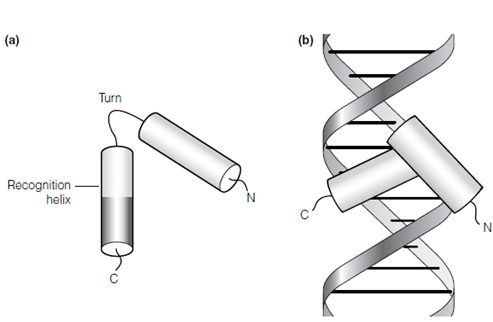
Figure: (a) Helix-turn-helix motif of a DNA-binding protein; (b) binding of the helix-turn-helix to target DNA showing the recognition helix lying in the major groove of the DNA.
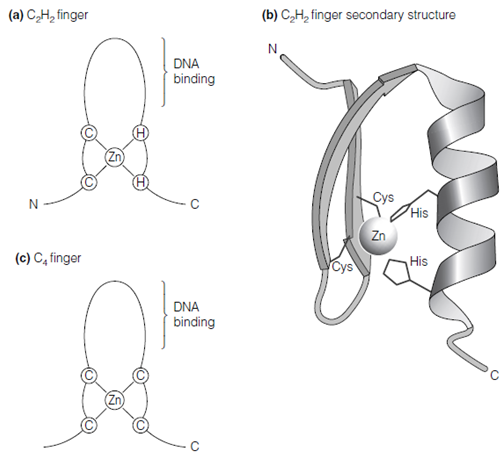
Figure: (a) A C2H2 zinc finger; (b) C2H2 zinc finger secondary structure. From A. Travers, DNA-Protein Interactions, Chapman & Hall, 1993. Reprinted with permission of A. Travers. (c) a C4 zinc finger.
The C4 zinc finger is also found in a number of transcription factors, involving steroid hormone receptor proteins. This motif forms a same structure to in which of C2H2zinc finger but has four cysteines coordinated to the zinc ion alter of two cysteines and two histidines that is described in the another figure.