Octahedral splitting
The five d orbitals with dissimilar values of the magnetic quantum number (m) contain similar energy in a free atom or ion. Though, in any compound, they interact in a different way with the surrounding ligands and a ligand field splitting is produced. The most common coordination is octahedral (Oh point group) along with six surrounding ligands (see Diagram). Then two of the d orbitals (dz2 and dx2-y2 well known together like the eg set) are observe at higher energy than the other three (dxy, dxz and dyz, known like t2g). Such type of a splitting (denoted Δo) takes place in any transition metal compound with octahedral coordination, involving aqua ions and several solids. Electronic transitions among t2g and eg orbitals give increase to colors, which are a well-known feature of transition metal complexes, and permit Δo to be measured experimentally.
Even though originally described in terms of electrostatic repulsion among d electrons and the ligands, now it is identified that ligand field splittings come from similar type of orbital overlap influences like donor-acceptor interactions
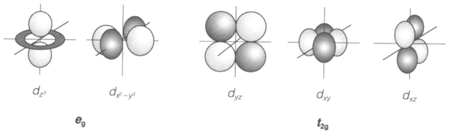
Fig. 1. The five d orbitals, showing eg and t2g sets in an octahedral complex, with ligands along the x, y and z axes.
Several ligands are coordinate to the metal ion using nonbonding electrons. A ligand lone-pair orbital pointing straight in the direction of the metal overlaps with the eg orbitals (1) but has the incorrect symmetry to interact along with t2g. The overlap provides rise to σ bonding and antibonding molecular orbitals (see diagram 2). The bonding orbitals are taken place through the electrons from the ligand, and it is the σ antibonding levels which create the 'metal' eg set, presented for the d electrons of the metal ion. A strong σ-donor ligand will give a large splitting Δo by increasing the eg energy, π bonding come into existence when ligands have orbitals directed perpendicular to the metal-ligand axis, that can interact with the metal t2g orbitals (2). Ligands like halide ions have occupied pπ orbitals and work as π-donors. This interaction increases the energy of the metal t2g orbitals, and get decreases Δo. Alternatively, π-acceptor ligands like CO have empty antibonding π orbitals. Overlap with the metal in this example causes the t2g orbitals to be lowered in energy that's why A0 is get increased.

The order of Δo values produced through different ligands is termed as the spectrochemical series. A partial series in order of the increasing splitting is:
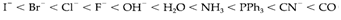
As supposed, strong a donor are usually high in the series, π donors are the low, and π-acceptor ligands like CN- and CO are between the highest, and termed as strong field ligands. The main trends with dissimilar metal ions are (i) Δo get increases with charge on the ion, and (ii) splittings are larger for 4d and 5d series elements than in the 3d series.
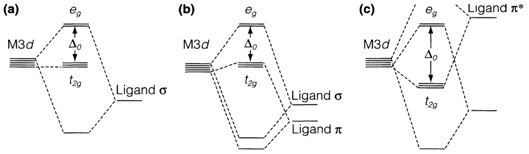
Fig. 2. Partial MO diagram showing an octahedral complex with (a) σ-donor only, (b) π-donor, and (c) π-acceptor ligands.
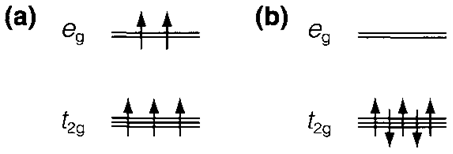
Fig. 3. Electron configurations for d5 in (a) high-spin and (b) low-spin octahedral complex.
Table 1. Electron configurations for dn high- and low-spin octahedral complexes, with corresponding ligand field stabilization energies
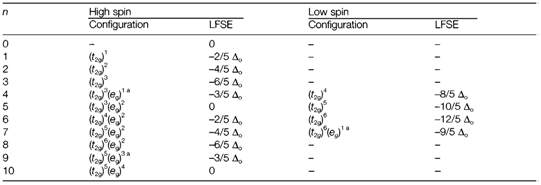
Configurations susceptible to Jahn-Teller distortion.