ATP synthase as a rotatory engine:
The ATP synthase can be seen as spherical projections from the inner membrane. If mitochondria are subjected to sonic disruption, submitochondrial vesicles are established in that the spheres of the ATP synthase point outward. In the year of 1960, Racker showed in which the spheres can be erased and in which the isolated spheres hydrolyze ATP, which is, the spheres have ATPase activity called F1. The exposed submitochondrial vesicles, devoid of the F1 ATPase, can since transport electrons along the electron transport chain but cannot synthesize ATP. These stripped submitochondrial vesicles contain the other main elements of the ATP synthase, known as F0 coupling factor 0 that spans the inner mitochondrial membrane. Because it is composed of these two main component parts, ATP synthase is also called F0F1 ATPase. The whole complex harnesses the energy released through electron transport to drive ATP synthesis while alone, without coupling to electron transport, the F1 parts hydrolyzes ATP.
The F1 ATPase consists of five kinds of polypeptides in the subsequent ratio: α3, β3, γ, δ, ε. The six α and β subunits are arranged alternately in a ring and with a middle stalk formed through the γ and ε subunits. The F0 consists of a ring of 10 to 14 c subunits sitting in the inner mitochondrial membrane. This contacts a single a subunit which links to two b subunits and the single δ subunit to establish a long column which connects to the head of the F1 ATPase. This whole structure, the F0F1 ATPase, is a remarkable molecular motor. In during ATP synthesis, protons flow by a channel build at the interface among the subunit and c subunits and this causes the c subunit ring to spin associatively to the static a subunit. Therefore the c ring acts as a 'rotor' and the subunit as a 'stator'. As
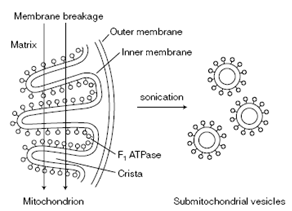
Figure: Sonic disruption (sonication) of mitochondria produces submitochondrial vesicles.
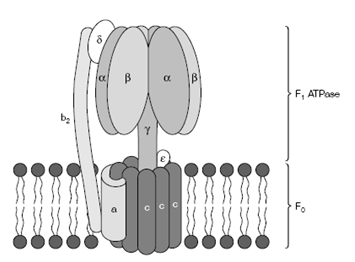
Figure: Schematic representation of the ATP synthase complex.
the c subunit ring rotates and it turns the γ subunit stalk, that thus turns rapidly inside the αβ ring. The αβ ring cannot rotate because it is held in place through the arm of the two δ subunit and b subunits.
The three β subunits in the αβ ring can bind inorganic phosphate and ADP. As the γ subunit rotates inside the αβ ring and the mechanical energy is used to drive ATP synthesis; that three molecules of ATP are synthesized for each 360 degree rotation of the γ subunit which equates to over 1000 molecules per second!. How is the ATP build? According to the binding-charge mechanism of Paul Boyer while the rotation causes the γ subunit to turn past the three β subunits and the protein conformation of the three nucleotide binding sites of the β subunits changes by variant states that causes ADP to be bound, then phosphorylated and then released as ATP So the mechanical energy of the rotating γ subunit is used to drive protein conformational modifies which in turn lead to ATP synthesis.