States of Matter
Matter can exist in one of the three states solid, liquid or gas. The physical state in which a matter exists is determined by the intermolecular forces of attraction between the constituting particles.
Properties of solids:
(i) Solids have a fixed shape and volume.
(ii) Intermolecular forcesof attraction between the particles of a solid are very strong.
(iii) The particles in a solid cannot move freely but vibrate in their own mean position.
(iv) The particles in a solid can be arranged in a regular pattern (crystal) or in a less ordered structure (amorphous).
(v) Solids usually have high density and melting and boiling point.
(vi) Solids cannot be squeezed / solids are incompressible.
(vii) They expand very little when heated.
(viii) A pure solid melts at a fixed temperature (melting point), presence of impurities raises their boiling point.
Properties of a liquid:
(i) Liquids have a fixed volume but no fixed shape; they take the shape of the container.
(ii) Intermolecular forces of attraction between the particles of a liquid are strong but not rigid.
(iii) Particles in a liquid can move freely within the body of the liquid, they slide across one another.
(iv) Liquids have very little intermolecular space and are incompressible.
(v) Liquids have surface tension.
(vi) The density of liquids is close to the of solids.
(vii) Liquids are called fluids, that is they can flow.
(viii) They show small expansions due to heat.
(ix) Pure liquids boils at a definite temperature (melting temperature), impurities increases their boiling point.
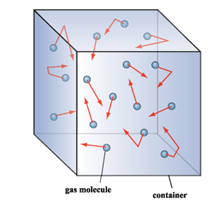
Properties of gases:
(i) Gases have no fixed shape or volume, they evenly fill up a closed container.
(ii) Intermolecular forces of attraction in gases are very weak.
(iii) Gas particles move randomly and freely in every direction at high speed.
(iv) There are large intermolecular spaces between gas particles.
(v) Gas particles move in the straight lines and change direction because of collision with another gas particle or with the walls of the container.
(vi) They have quite low density.
(vii) Gases show the property of large expansions when heated.
The kinetic theory of matter:
The kinetic theory of matter explains the macroscopic properties observed in different states of matter. The theory supposes that the matter is made up of small particles, which are in the constant random motion, and therefore the particles collide with each other and the collisions are perfectly elastic.
In solids these particles are held closely together by very strong electrostatic forces of attraction. Thus the particles are not able to move freely and can vibrate in a fixed position. When a solid is heated particles gains the kinetic energy and begins to vibrate fasterand faster. At one point these particles vibrate so much that they are able to break free from attractive forces which hold them as a solid. The particles move freely and the solid turns into a liquid.
In a liquid the particles can freely move within the body of liquid. As a liquid is heated its temperature increases and the particles gains the kinetic energy and moves faster and faster. At a certain temperature (boiling point) the particles have enough kinetic energy to overcome the intermolecular attraction that holds them as liquid and escapes from the body of the liquid and turns into a gas (boiling).
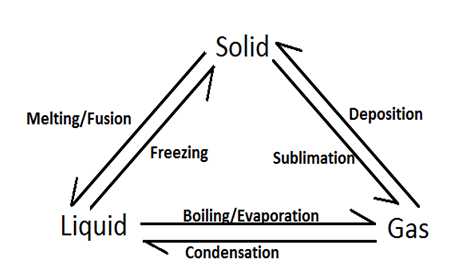
Interconversion of states of matter is illustrated in the figure drawn below:
www.expertsmind.com - States of Matter Assignment Help, States of Matter Homework Help, States of Matter Assignment Tutors, States of Matter Solutions, States of Matter Answers, Chemistry Assignment Tutors