Separation techniques
When we perform an experiment, we end up with a mixture of substances rather than only one. We should know how to separate the mixtures.
A single substance that has no other substances mixing with it is known as PURE SUBSTANCE. If there is any thing else mixed with it, it is a mixture.
Solid/Liquid Mixtures
The mixture of dissolved salts in water is an example of a solid/liquid mixture. The mixture is very much clear and no salt can be seen. This sort of mixture is called as solution. The solid which dissolves, such as salt, is called as solute. The liquid that solute dissolves, like water, is called as solvent. Solids like sugar and salt that dissolve are describe as soluble. Solids like mud and coin which do not dissolve are described as insoluble.
SOLUTE + SOLVENT = SOLUTION
We say that a solution which contains a little solute in a given amount of solvent is dilute. A solution contains a lot of solute in a given amount of solvent called as CONCENTRATED.
We a solution w can dissolve all the solute is called as SATURATED. Generally cold solvents dissolve less solute than hot solvents.
A saturated solution is the solution which has dissolved all solute, at the given temperature.
Water is the common solvent; but there are many other solvents which we used in industry and around houses. They are all required to dissolve substances which cannot be dissolved in water.
Suspensions
At times it is difficult to distinguish between a cup of PURE water and a cup of salt in water mixture just by looking, but there are times we can simply recognize a mixture. For instance, by looking at the chalk in water in the diagram drawn below. We can see there are white specks in water, as the chalk cannot dissolve in water. The chalk particles are suspended in water. They are so light and they will not sink. We call this sort of mixture a suspension. Insoluble solid particles are suspended throughout the liquid in process of suspension. They are in groups in which they are large enough to be seen.
Liquid/Liquid Mixtures
We used to word MISCIBLE when the liquids mixed together completely in order to form one single liquid. We called those liquids which cannot mix together completely as IMMISCIBLE. Oil and water form 2 separate layers when they are mixed togather.
The reason which we need to pure substance is because in Chemistry, pure substances are needed to produce drugs or perform experiments in the laboratory. However, most substances obtained from nature are mixtures. Therefore these mixtures need to be separated and purified before we can use them.
Methods of Purification and Seperation
Separation and Purification of substances are important techniques in Chemistry. Some of the common methods of purification and separation are explained below.
Decantation
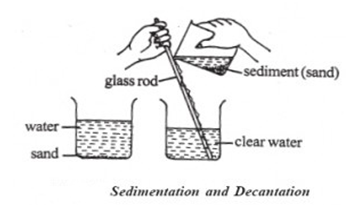
Decantation is a very quick method for separating a mixture of a liquid and a heavier solid.
To separate the mixture of water and sand, first, we should let the sand to settle on the bottom of the container (sedimentation). Then we pour off the water at the top into the other container.
This cannot be used to separate the mixture of a liquid and a light sold, like chalk in water. The particles of chalk are suspended in water. They are so light that they do not sink down to bottom for a long time.
Centrifugation
Centrifugation is used to separate the suspension. The suspension of solid in liquid is poured into a centrifuge tube, then spin around fast in a centrifuge. The spinning motion forces the solid to the bottom of the tube. Then the liquid can be poured off from the solid.
Filtration
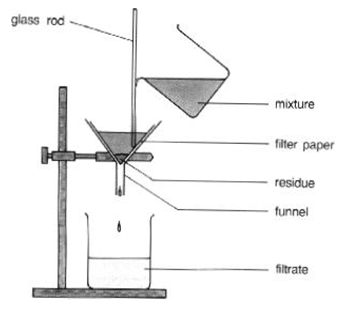
This is a process which is especially effective for separating suspensions, for instance mud in water. We pour the mixture into the funnel fitted with a piece of filter paper. There are tiny holes in filter paper for the liquid to pass through, the solid particles are too large to do so, and thus the solid particles will stay on the paper called as solid RESIDUE. The liquid which pass through it is called as FILTRATE.
Evaporation
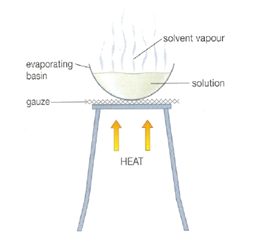
We cannot separate the mixture which is a solution using filtration or centrifugation. As it is spread all over the solvent in tiny particles. The solution is heated such that the solvent evaporates, and leave the solid behind. The diagram below show by using this method, salt can be obtained from the solution of it. Only solute can be obtained, and solvent will evaporate away in the process of EVAPORATION.
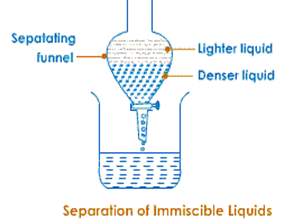
Seperating Funnel
A separating funnel will be used to separate 2 immiscible liquids. The mixture is poured in separating funnel and left undisturbed for sometime so that the liquids form 2 distinct layers. The tap is then opened and the denser liquid is collected in the beaker. The tap is closed as the separating layer reaches the tap and then the lighter liquid is collected in the other container.
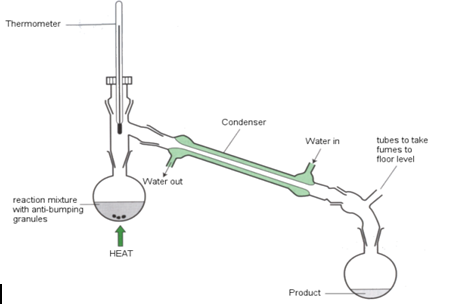
Simple Distillation
This method can be used to separate a solution and collect the solute and the solvent both. The apparatus is set up as shown in figure below. The flask contains the solution to be separated with some anti-bumping granules for the smooth boiling. When it gets heated the solvent evaporates and the vapour travels into the condenser where it is converted back to liquid and is collected in a beaker. The solute stays in flask.
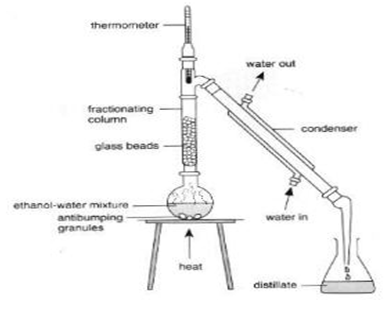
Fractional Distillation
It is used to separate the mixture of t2wo miscible liquids, for instance to separate a mixture of ethanol and water, by using the difference in their boiling points. The apparatus is set up as shown in figure below. The flask contains the mixture with a thermometer to monitor the temperature of vapour passing. As flask is heated the liquid having lower boiling point (alcohol) will boil 1st. It is essential to keep the temperature of thermometer near the boiling point of alcohol (780C) to make sure that no water gets evaporated. If the temperature tends to increase above 780C the Bunsen burner is removed and replaced when the temperature decreases.
When the flask is heated alcohol gets boiled off 1st and its vapour enters the condenser to be condensed back to liquid which is then collected in a beaker. Any water which is evaporated is condensed in contact with the glass beads in the fractionating column and falls back to flask.
Therefore water and alcohol is separated.
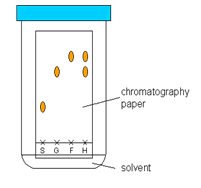
Chromatography
The technique of separating two or more colors from a mixture of colors is known as chromatography. 1st a rectangular filter paper is taken and a base line is drawn near one end of the paper with the pencil. Spots of the mixtures of the colors are put on the base line. Then the paper is hung over an organic solvent, such as alcohol, in such a way so that the edge of the paper is touching the solvent. It should be ensured that the base line never touches solvent.
The solvent will travel up the paper and on its way it will dissolve the mixtures of colors. As the mixtures of colors travels up they will be separated. Because different compounds have different affinity (adsorption) for the filter paper. Those with higher adsorption will spread slower than those with lower adsorption. Thus the colours will be separated.
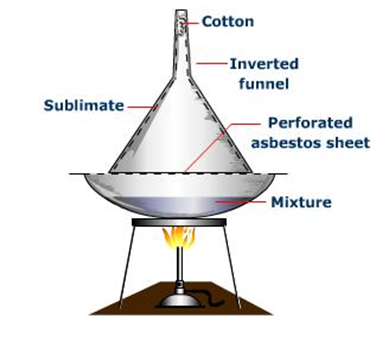
Sublimation
Few substances such as naphthalene, iodine, ammonium salts can sublimate. Sublimation can be used if any substance is contaminated or spoiled by any of the sublimates.
The mixture is taken on a watch glass and a funnel is held over it in the inverted position. The mixture is heated. The sublimate will sublime and will solidify again in contact with inverted funnel held above. Therefore the 2 substances will be separated.