IUPAC system of nomenclature of complex compounds
The naming of any organic compound depends on the name of normal parent hydrocarbon from which it has been derived. IUPAC system has framed a set of rules for various types of organic compounds.
Rules for Naming complex aliphatic compounds when no functional group is present (saturated hydrocarbon or paraffins or Alkanes)
(i) Longest chain rule : The first step in naming an organic compound is to select the longest continuous chain of carbon atoms which may or may not be horizontal (straight). This continuous chain is called parent chain or main chain and other carbon chains attached to it are known as side chains (substituents). Examples :
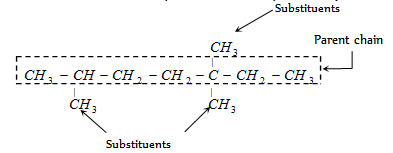
If two distinct chains of similar length are possible, the chain with maximum number of side chains or alkyl groups is selected.
(ii) Position of the substituent : Number of the carbon atoms in the base chain as 1, 2, 3,....... etc. initiating from the end which gives lower number to the carbon atoms carrying the alternative. As given,
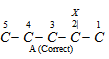
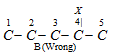
The number that shows the position of the substituent or side chain is called locant.
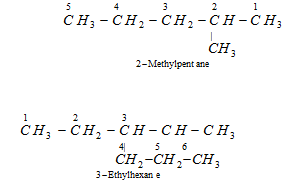
(iii) Lowest set of locants : When two or more substituents are present, then end of the parent chain which gives the lowest set of the locants is preferred for numbering.
This rule is called lowest set of locants. That defines that when two or more different sets of locants are possible, that set of substitutes which when related term by term with other parts, each in order of increasing value, has the minimum term at the first point of difference. This principle is needed irrespective of the nature of the alternative. As shown,
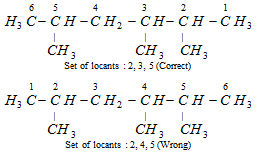
The correct set of substituents is 2, 3, 5 and not 2, 4, 5. The first part is lower than the second set because at the first difference 3 is less than 4. 2 and the first difference is with the second locant 3, 4. We can relate term by term as 2-2, 3-4 (first difference), 5-5. Only first point of difference is considered for preference. Similarly for the compounds,
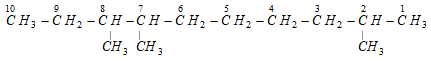
Set of locants : 2, 7, 8 (Correct)
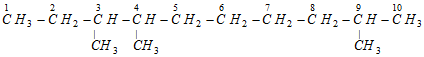
Set of locants : 3, 4, 9 (Wrong)
First set of locants 2, 7, 8 is lower than second set 3, 4, 9 because at the first point of difference 2 is lower than 3.
Lowest sum rule : It may be noted that earlier, the numbering of the parent chain containing two or more substituents was done in such a way that sum of the locants is the lowest. This rule is called lowest sum principle. For example, the carbon chain of alkanes given below should be numbered as indicated in structures A and not according to structure B.
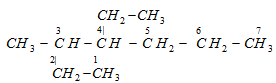 ;
;
A (correct) Sum of locants =3+4=7
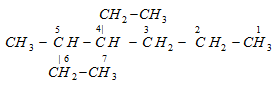
B (wrong) Sum of locants =4+5=9
According to latest IUPAC system of nomenclature, the lowest set of locants is preferred even if it violates the lowest sum rule. For example,
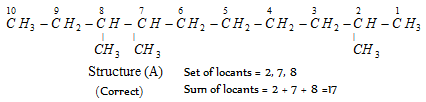
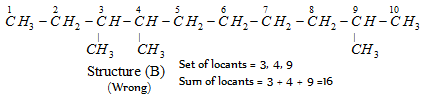
This compound is numbered as 2, 7, 8 and not as 3, 4, 9 in accordance with latest lowest set of locants rule, even though it violates lowest sum rule.
(iv) Presence of more than one same substituent : If the same substituent or side chain happens more than once, the prefixes tri, tetra, di ..........etc., are attached to the names of the locants. As given,
(v) Naming different substituents : If two or more different substituents or side chains are present in the molecule, they are named in the alphabetical order along with their appropriate positions.
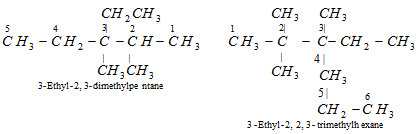
(vi) Naming different substituents at equivalent position : In case, there are different alkyl substituents at equivalent positions, then numbering of the parent chain is done in such a way that the alkyl group which comes first in the alphabetical order gets the lower number.
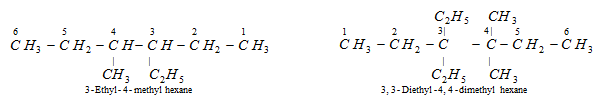
(vii) Naming the complex substituents (or substituted substituents) : If the substituent on the parent chain is complex (i.e. it is branched) it is named as substituted alkyl group by numbering the carbon atom of this group attached to the parent chain as 1. The name of such substituent is given in brackets in order to avoid confusion with the numbering of the parent chain. For example,
The name of the hard substituent is always written in brackets.
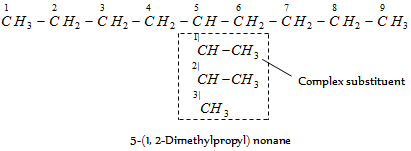
While deciding the alphabetical order of the various substituents, the name of the hard substituent is taken to begin with the first letter of the entire name. It can be remembered that in case of simple substituents, however, the multiplying prefixes are not considered. The names of simple substituents are first alphabetized and then multiplying prefixes are inserted. As given,
It may be noted that dimethyl propyl (a complex substituent) is alphabetized under d and not under m. Therefore, it is cited before ethyl (e).
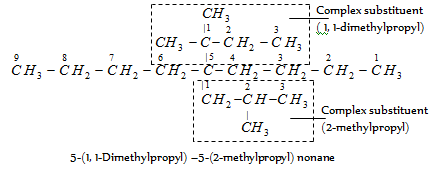
The substituent dimethyl is cited first because it is alphabetized under d. Similarly,
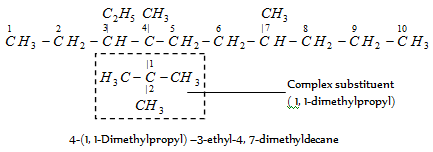
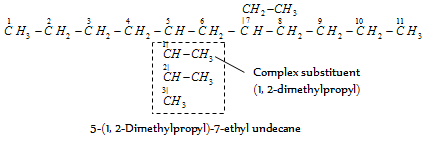
When the names of two or more complex substituents are composed of identical words, priority for citation is provided to the substituent which has lowest locant at the first cited point of difference within the complex substituent. For example,
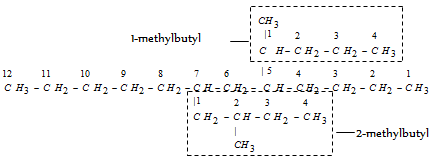
- 5(1-methyl butyl)-7-(2-methyl butyl) dodecane
The substituent (1-methylbutyl) is written first because it has lower locant than the substituent (2-methylbutyl).
When the similar complex substituent (substituted in the same way) occurs more than once, it is indicated by the multiplying prefix bis (for two), tris (for three), tetra kis (for four) etc.
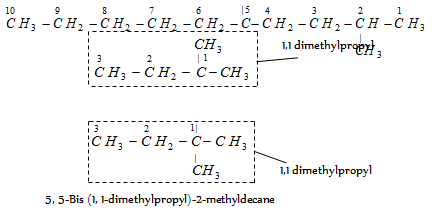
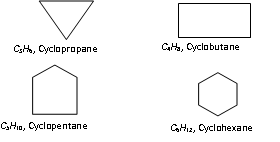
(viii) Cyclic hydrocarbons : These compounds contain carbon chain skeletons which are closed to form rings. The saturated hydrocarbons with ring of carbon atoms in the molecule are called cycloalkanes. These have the general relation CnH2n.
The cyclic element is called by prefixing cyclo to the name of the corresponding straight chain alkane. For example,
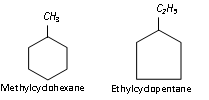
If side chains are present, then the rules given in the previous section are applied. For example,
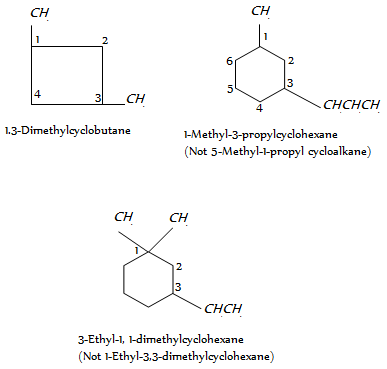
When more than one side chains are present, the numbering is done beginning with one side chain so that the next side chain gets the lower possible value. As given,
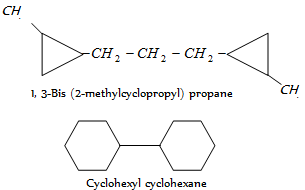
When a single ring system is attached to a single chain with a greater number of carbon atoms or when more than one ring system is attached to a single chain, then it is named as cycloalkylalkanes. As given,
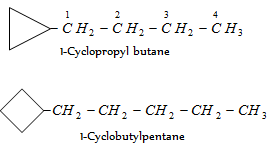
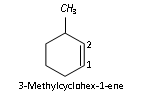
In case of substituted cycloalkenes, the double bond is given the lowest possible number and numbering is done in such a way that the substituents get the lowest number.
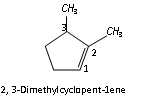
According to the IUPAC system of Nomenclature, certain trivial or semi- systematic names may be used for unsubstituted radicals. For example, the following names may be used,
Table :
|
Order of preference
|
Preflx
|
Suffix (ending)
|
|
- SO3H
|
Sulpho
|
Sulphonic acid
|
|
- COOH
|
Carboxy
|
- oic acid
|
|
- COOR
|
Alkoxy carbonyl
|
Alkyl alkanoate
|
|
- COX
|
Haloformyl
|
Oyl halide
|
|
- CONH2
|
Carbamoyl
|
- amide
|
|
- C º N
|
Cyano
|
- nitrile
|
|
- CHO
|
Formyl
|
- al
|
|
> C = O
|
Keto
|
- one
|
|
- OH
|
Hydroxy
|
- ol
|
|
- NH2
|
Amine
|
- amine
|
|
C = C
|
-
|
- ene
|
|
- C ≡ C -
|
-
|
- yne
|
|
- O -
|
Epoxy
|
-
|
|
- X
|
Halo
|
-
|
|
- NO2
|
Nitro
|
-
|
However, when these are substituted, these names cannot be used as such. As given,
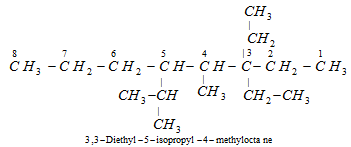
It may be noted that while writing the substituent's name in alphabetical order, the neo -and prefixes iso -are considered to be part of the fundamental name. However, the prefixes sec-and tert-are not considered to be the part of the fundamental name.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with IUPAC system of nomenclature of complex compounds questions? IUPAC system of nomenclature of complex compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their IUPAC system of nomenclature of complex compounds related problems. We provide step by step IUPAC system of nomenclature of complex compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for IUPAC system of nomenclature of complex compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours