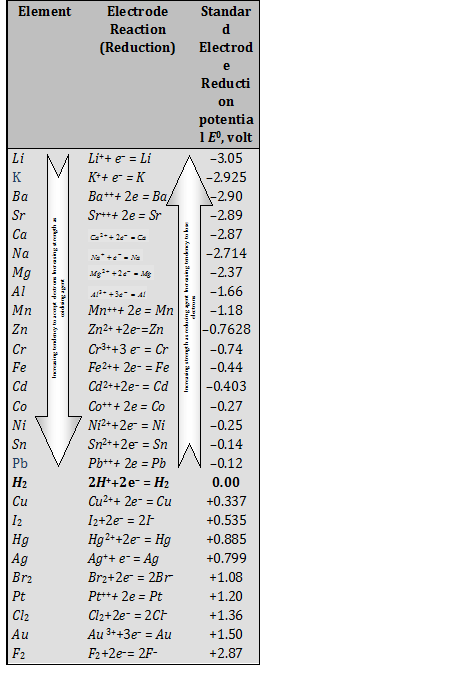
Electrochemical series
(1) The standard reduction potentials of a large number of electrodes have been measured using standard hydrogen electrode as the reference electrode. These several electrodes can be arranged in the increasing or decreasing order of their reduction potentials. Arrangements of the elements in order of increasing reduction potential values are known as electrochemical series. It is also known as activity series, of a few typical electrodes.
(2) Characteristics features of Electrochemical series
(i) The negative sign of the standard reduction potential indicates that an electrode when joined with SHE acts as anode and oxidation occurs on this electrode. For instance, standard reduction potential of zinc is -0.76
volt, When zinc electrode is joined with SHE, it acts as anode (-ve electrode) that is oxidation takes place on this electrode. Similarly, the +ve sign of standard reduction potential indicates that the electrode when joined with SHE acts as cathode and reduction occurs on this electrode.
(ii) The substances, which are stronger reducing agents than hydrogen are placed above hydrogen in the series and have negative values of standard reduction potentials. All the substances which possess positive values of reduction potentials and placed below hydrogen in the series are weaker reducing agents than hydrogen.
(iii) Substances, which are stronger oxidizing agents than H+ ion are placed below hydrogen in the series.
(iv) The metals on the top (having high negative value of standard reduction potentials) have the tendency to lose electrons readily. These are usually active metals. The activity of metals decreases from top to bottom. The non-metals on the bottom (having high positive values of standard reduction potentials) have the tendency to accept electrons readily. These are active non-metals. The activity of non-metals increases from top to bottom.
Table: Standard reduction electrode potentials at 298K
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Electrochemical series questions? Electrochemical series topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Electrochemical series related problems. We provide step by step Electrochemical series question's answers with 100% plagiarism free content. We prepare quality content and notes for Electrochemical series topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours